List Five Transition Metals and Describe a Use for Each
Three of the most noteworthy elements are iron cobalt and nickel as they are only elements known to produce a magnetic field. Sometimes included in the transition metal group are the lanthanides and actinides.

Lesson Explainer Electronic Configurations Of Transition Metals Nagwa
With a small amount of ammonia hydrogen ions are pulled off the hexaaqua ion to give the same neutral complex.
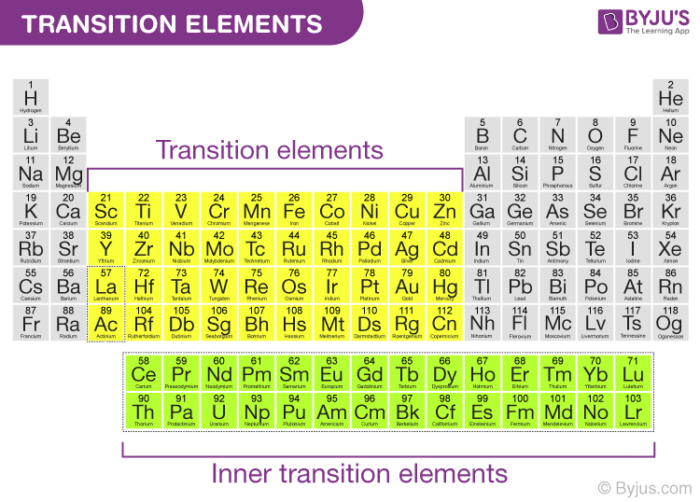
. What elements are transition metals. List three properties of transition metals that are different from the metals in group 1 the alkali metals. The transition metals as a group have.
Cobalt nickel platinum and other metals are employed as catalysts in a number of chemical reactions. These three main transition series are included in the set of 30 elements often called the d -block transition metals. Copper a transition metal is widely used in electrical wiring because of its high tensile strength malleability ductility and electrical conductivity.
Some of the transition metals have widespread usage particularly iron and steel for construction and copper for electrical wiring and water pipes. Transition metals look shiny and metallic. 1 Ion changes OS gains or loses electrons to form intermediates with lower activation energies.
Some of the more well-known transitional metals include titanium iron manganese nickel copper cobalt silver mercury and gold. However they are also considered as transition metals because they have similar properties to those of transition metals. Co H2O62 aq 2NH3 aq --- Co H204 OH2 s 2NH4 aq That precipitate dissolves if you add an excess of ammonia.
There are several properties identified in the transition elements that are not found in other elements as a result. Transition metals are kinds of metals that have electrons in d or f orbitals. Transition metals are applied in the organic reactions.
A metal-to ligand charge transfer MLCT transition will be most likely when the metal is in a low oxidation state and the ligand is. The second series includes the elements yttrium symbol Y atomic number 39 to cadmium symbol Cd atomic number 48. Transition metals are like main group metals in many ways.
Name the Transition Metal. The d10 metals namely Zn Cd and Hg have completely filled d -orbitals. The ammonia replaces water as a ligand to give.
Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. Transition metals are in columns 3-12 of the periodic table and in the lanthanide and actinide series. The ammonia acts as both a base and a ligand.
Hence the transition metals are extremely important elements. 2 Ways Transition Metals Behave as Catalysts. They also are good conductors of heat and electricity.
In other words they have d1 to d9 electrons. Thus the electronic configurations and the properties of the transition metals are briefly discussed in this article. Many of the metals are used in electronics while others such as gold and silver are used in monetary systems.
In each case the metals Cr and Mn have oxidation states of 6 or higher. Transition metals often form important alloys. Iron is a versatile structural material.
These include platinum gold and silver. These metals tend to be very hard. Atomic Number 22 -.
They are also hard metals that conduct heat and electricity well. There are a number of elements that are classified as transition metals. Transition metals are conductors of electricity possess high density and high melting and boiling points.
The three main differences are. Transition metals complex under goes a series of reactions that are generally unlike those main group compounds. They look like metals they are malleable and ductile they conduct heat and electricity and they form positive ions.
Haber Process Equation Use of Product. 2 Metal provides a surface for reactants to adsorb to holding them in place. The transition metals consist of 38 elements in the periodic table situated in the groups 3-12.
Atomic Number 21 - Sc. For more detail click on the individual individual elements. The most fundamental is the simple coordination and dissociation of ligands.
These elements are well-known for their various oxidation states which is possible due to the presence of. The transition metals are malleable easily hammered into shape or bent. Transition metals are both ductile and malleable and usually lustrous in appearance.
Scandium forms 25 ppm of the earths crust cf the better known transition metals cobalt at 29 ppm and copper at 68 ppm. Different phase to reactants. Name the Transition Metal.
These include variable oxidation state oxidation number complex ion formation coloured ions and catalytic activity. This page explains what a transition metal is in terms of its electronic structure and then goes on to look at the general features of transition metal chemistry. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table.
For example iron in steel zinc and copper in brass. The third series extends from lanthanum symbol La atomic number 57 to mercury symbol Hg atomic number 80. The fact the two best conductors of electricity are a transition metal copper and a main group metal aluminum shows the extent to which the physical properties of main group metals and.
Because there are so many metals in this group there are a wide variety of uses. You will find some of this covered quite briefly on this page. Compounds of many transition elements are distinctive for being widely and vividly colored.
They are called the inner transition. They occupy columns 3 through 12 of the periodic table and include such metals as titanium copper nickel silver platinum and gold. The application of transition metals is as follows.
They appear in almost every facet of our day to day life from electric cabling to decorative door handles to corrosion-resistant alloys. Transition metals often make good catalysts for particular. Because of this unique filling order the transition elements are often referred to as d -block elements.
The properties of individual transition metals determine which. Transition metals are the d -block elements and they have incompletely filled d -orbitals.

Transition Elements General Properties And Trends With Faqs

Transition Metal Definition Properties Elements Facts Britannica
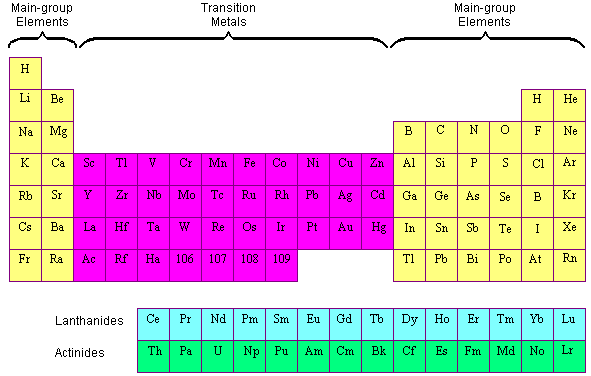

No comments for "List Five Transition Metals and Describe a Use for Each"
Post a Comment